The use of cannabinoids containing plant extracts as herbal medicine can be traced back to as early as 500 BC. In recent years, the medical and health-related applications of one of the non-psychotic cannabinoids, cannabidiol or CBD, has garnered tremendous attention. In this review, we will discuss the most recent findings that strongly support the further development of CBD as a promising anti-cancer drug.
Recently, cannabinoids, such as cannabidiol (CBD) and Δ9-tetrahydrocannabinol (THC), have been the subject of intensive research and heavy scrutiny. Cannabinoids encompass a wide array of organic molecules, including those that are physiologically produced in humans, synthesised in laboratories, and extracted primarily from the Cannabis sativa plant. These organic molecules share similarities in their chemical structures as well as in their protein binding profiles. However, pronounced differences do exist in their mechanisms of action and clinical applications, which will be briefly compared and contrasted in this review. The mechanism of action of CBD and its potential applications in cancer therapy will be the major focus of this review article.
Keywords: cannabinoids, cannabidiol, CBD, anti-cancer drug
The use of Cannabis sativa plant extract as herbal medicine can be dated back as early as 500 BC in Asia. The human endocannabinoid system was uncovered after the discovery of the cannabinoid receptors. It was initially thought that cannabinoids produce their physiological effects via nonspecific interactions with the cellular membrane; however, research involving rat models in the late-1980s led to the discovery and characterization of specific cannabinoid receptors, CB1 and CB2. The CB1 receptor is expressed throughout the central nervous system (CNS), whereas the CB2 receptor is found primarily in the immune system and hematopoietic cells. Soon after the discovery of CB1 and CB2, their endogenous ligands, or endocannabinoids, were also identified, including 2-arachidonolyglycerol (2-AG) and N-arachidonoylethanolamine (AEA, also called anandamide) (Figure 1A, i and ii). CB1 and CB2 belong to a large family of transmembrane proteins, called G protein-coupled receptors (GPCRs), and are now believed to be responsible for the majority of the physiological effects of the endocannabinoids (Figure 1B). Both receptors are coupled with Gαi/o, which can inhibit the adenylyl cyclase (AC). CB1 can also be coupled to Gαq/11 and Gα12/13. CB2 has also been shown to act through Gαs. For a more in-depth understanding of the downstream effects of the endocannabinoids and their receptors under physiological conditions, we refer you to other excellent reviews on the topic.
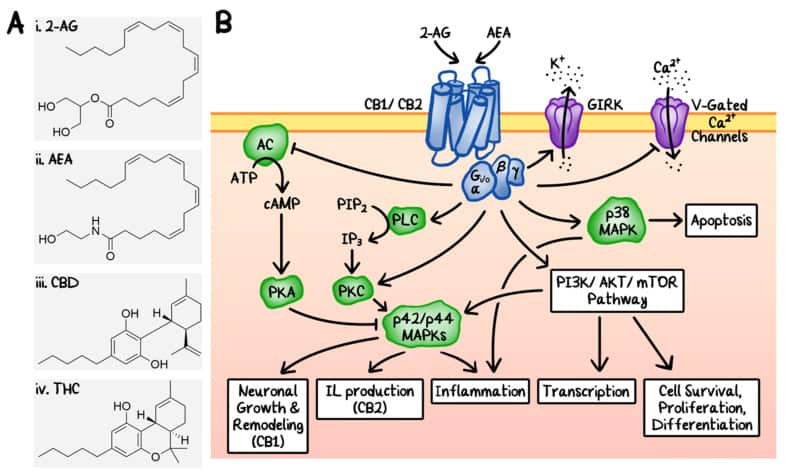
Figure 1
Endocannabinoid system. (A) Chemical structures of two endogenous cannabinoids, 2-arachidonylglycerol (i, 2-AG) and N-arachidonylethanolamine (ii, AEA), and two representative exogenous cannabinoids from Cannabis sativa, cannabidiol (iii, CBD) and Δ9-tetrahydrocannabinol (iv, Δ9-THC). (B) Schematic diagrams of the signalling transduction pathways of the endocannabinoid system. 2-AG and AEA are agonists of CB1 and CB2. Some of the downstream effects include: (1) upregulation of p42/p44 mitogen-activated protein kinases (MAPKs) by direct inhibition of adenylyl cyclase (AC) and direct activation of phospholipase C (PLC), leading to the induction of neuronal growth, interleukin production, and inflammation. PKA: protein kinase A. PKC: protein kinase C. (2) Activation of p38 MAPK, which induces inflammation and apoptosis. (3) Activation of the phosphatidylinositol-3-kinase (PI3K)/AKT and the mammalian target of rapamycin (mTOR) signalling pathways. Under certain conditions, these endocannabinoids can also induce transcription, cell survival, proliferation, and differentiation through similar pathways. Additionally, the cannabinoid receptors can also modulate ion channels including G protein-coupled inwardly-rectifying potassium channels (GIRKs) and voltage (V)-gated calcium channels.
The two primary endocannabinoids, 2-AG and AEA, can activate either CB1 or CB2 and are synthesised on-demand from phospholipid precursors in response to an elevation of intracellular calcium. In addition to CB1 and CB2, 2-AG and AEA can also bind other transmembrane proteins, including orphan G protein-coupled receptor 55 (GPR55), peroxisome proliferator-activated receptors (PPARs), and transient receptor potential vanilloid (TRPV) channel type 1 (TRPV1).
The TRPV channels are of particular interest concerning the anti-tumor functions of cannabidiol (CBD) (Figure 1A, iii), which will be discussed in more detail later. Six different TRPV channels have been identified in humans and can be subdivided into two groups: TRPV1, TRPV2, TRPV3, and TRPV4 belong to group I, while TRPV5 and TRPV6 fall into group II. Though the exact functions of the TRPV channels are still under intense investigation, they are likely involved in regulating cellular calcium homeostasis. For example, TRPV1 and TRPV2 can be found in the cytoplasmic membrane as well as the endoplasmic reticulum (ER) membrane. They both play an important role in regulating the cytoplasmic calcium concentration from the extracellular sources as well as the calcium stored within the ER. Disruption of cellular calcium homeostasis can lead to increased production of reactive oxygen species (ROS), ER stress, and cell death.
A variety of cannabinoids exist in the Cannabis sativa plant (also known as the hemp or marijuana plant). There are more than 100 different cannabinoids and Δ9–tetrahydrocannabinol (Δ9-THC) (Figure 1A, iv) and CBD are the most well-known ones. The so called drug-type Cannabis sativa contains higher level of Δ9-THC and is used more widely for medical and recreational purposes, whereas the fibre-type cannabis contains less than 0.2% of Δ9-THC and is more often used in textiles and food. Δ9-THC is thought to be the psychotic cannabinoid and many of its psychoactive effects are due to its interaction with the CB1 receptor, whereas its immune-modulatory properties are likely due to its interaction with the CB2 receptor. In contrast, CBD is non-psychoactive and has a relatively low affinity to both CB1 and CB2.
The utility of cannabinoids in the treatment of cancer has long been of great interest. Recently, both CB1 and CB2 were found to be expressed in many cancer types. Intriguingly, both receptors were often undetectable at the site of the cancers’ origin before neoplastic transformation. Additional evidence for the role of endocannabinoid system in neoplasia came when Wang and colleagues showed that CB1 has a tumour-suppressive function in a genetically modified mouse model of colon cancer. On the other hand, CB1 is upregulated in hepatocellular carcinoma and Hodgkin lymphoma, and the extent to which CB1 was overexpressed correlated with disease severity in epithelial ovarian carcinoma. Similarly, CB2 has also been found to be overexpressed in HER2+ breast cancers and gliomas. Finally, it was shown that overexpression of both CB1 and CB2 was correlated with poor prognosis in stage IV colorectal carcinoma. In 1976, Carchman and colleagues found that the administration of cannabinoids, such as Δ8-THC, Δ9-THC, and CBD, inhibited the DNA synthesis and growth of lung adenocarcinoma in cultured cells as well as mouse tumour models. Similar effects were seen in both in vitro and in vivo models of various other cancers, including glioma, breast, pancreas, prostate, colorectal carcinoma, and lymphoma. There are various proposed mechanisms of action behind these findings, including, but not limited to: cell cycle arrest, induction of apoptosis, as well as inhibition of neovascularization, migration, adhesion, invasion, and metastasis. Despite the multitude of positive results with Δ9-THC-related cannabinoids in cancer research, the clinical use of these compounds is limited due to their psychoactive side effects.
In contrast to the Δ9-THC-related cannabinoids, CBD has no known psychoactive effects, and therefore, has recently been the focus of intense research in many therapeutic areas, including cancer. At present, the Food and Drug Administration (FDA) has only approved Epidiolex, purified CBD, for use in patients with seizures associated with the Lennox-Gastaut syndrome or Dravet syndrome. Globally, more than 40 countries have approved medical marijuana/cannabis programs, whereas this is true of 34 states in the USA, plus the District of Columbia, Guam, Puerto Rico, and US Virgin Islands. While marijuana is considered a Schedule I controlled substance in the US, the Drug Enforcement Administration ruled that CBD is a Schedule V controlled substance. When approved by the FDA, CBD must contain less than 0.1% of Δ9-THC.
It has been noted that CBD has a relatively low affinity to both CB1 and CB2. However, it was found that CBD can act as an antagonist to CB1 in the mouse vas deferens and brain tissues in vitro. There is also evidence suggesting that CBD may act as an inverse agonist of human CB2. Other cellular receptors that CBD may interact with include TRPVs, 5-HT1A, GPR55, and PPARγ. It has been hypothesised that CBD has robust anti-proliferative and pro-apoptotic effects. In addition, it may also inhibit cancer cell migration, invasion, and metastasis. The utility of CBD in anti-tumor therapy and the potential mechanisms behind it will be discussed in more detail below. Since much of the anti-tumor activity of CBD seems to hinge on its regulation of ROS, ER stress, and immune modulation, we will first summarise the interplays between ROS, ER stress, and inflammation and their known effects on various aspects of tumorigenesis. Thereafter, we will further discuss the anti-tumor effects of CBD on a variety of cancers and the molecular mechanisms behind them.
read more at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7693730/